|
2023 Annual Conference and HCSRN State of the Network Update
|
|
|
Governing Board Chair, Michael A. Horberg, MD, MAS, FACP, FIDSA, Associate Medical Director, Kaiser Permanente Mid-Atlantic Permanente Medical Group and Executive Director,
Mid-Atlantic Permanente Research Institute
and
Suzanne Simons, MS, Interim Executive Director
On February 21, the 29th Annual Conference of the HCSRN kicked off at the Sheraton Downtown Denver with more than 320 participants from 20 HCSRN member institutions. Attendees were happy to gather again, in person, to reconnect and network during the two and a half-day conference.
Claudia Steiner, MD, MPH, Executive Director, Institute for Health Research, Kaiser Permanent Colorado (KPCO), and Professor, Health Systems Science, Kaiser Permanente School of Medicine welcomed the attendees to the Mile High City. Although the weather was cold, the warm hospitality extended by Claudia and her team set the perfect tone for the meeting.
Abdul Shaikh, PhD, MSHc, Global Leader for Population Health Analytics for Amazon Web Services provided the opening plenary session with his presentation, Leveraging the Power of the HCSRN Network to Accelerate Progress on Health Equity. Using examples from his career spanning government, academia, and industry—including HCSRN member organizations, he shared insights and learnings from new initiatives and approaches to accelerate progress on health equity for clinical translational research.
Each day throughout the conference, attendees were encouraged to take advantage of network opportunities during breakfast and lunch, ancillary meetings, informal gatherings, the opening reception, and poster sessions.
Governing Board Chair, Michael Horberg, MD, MAS, shared many exciting updates during the State of the Network (SON) address on Wednesday, February 22. Recognizing the visionary leaders who founded HCSRN in 1994, Dr. Horberg also expressed his deep appreciation for the leadership of Immediate Past Chair, Robert Greenlee, PhD, MPH. Last year, Dr. Greenlee orchestrated the transition of HCSRN to Capitol Hill Management Services, headquartered in Albany, NY. Now a designated 501(c)3 non-profit organization, HCSRN has increased capacity and resources to further the HCSRN mission.
For the remainder of 2023, the organizational focus will include:
- Engagement => Value for participation
- Collaboration => Funded opportunities
- Sustainability => Organizational Transformation/New Central Team
- VDW operations => Working group leads and new data development
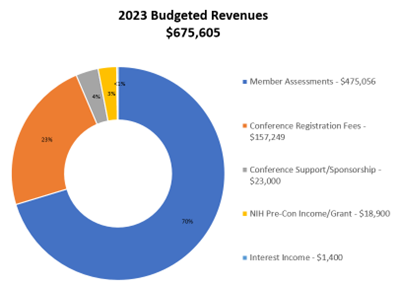
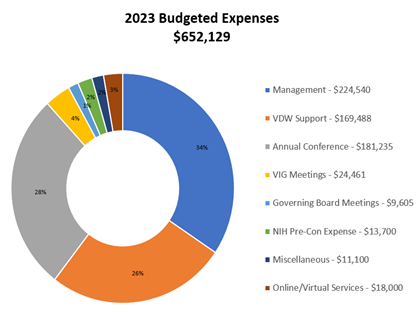
The HQ3: A Bridge Plan to Our Future was reviewed. This transition plan ensures that HCSRN accomplishes everything it should during the conversion from an unincorporated network to a professional 501(c)3 membership association (our research institutions) while providing quality membership services, programming, and networking opportunities. Dr. Horberg shared the 2023 Budgeted Revenue and Expense numbers which demonstrated a nearly $25,000 surplus, to be used for furthering the mission and goals of the organization.
|
 |
 |
|
The VDW update included that a new SDOH table is planned, and Stacey Honda, MD from Kaiser Permanente Hawaii, is serving as the VIG Board Liaison following the retirement of Lois Lamerato (see Transition article). A heartfelt thank you was extended to Mark Jurkovich, Reesa Laws, Celeste Machen, and the workgroup leads for their tireless work on behalf of the organization.
The public facing HCSRN website was debuted on March 1 with a new look. There is still work to be done to update posted documents, but the sleek design and navigation system convey the evolution of HCSRN. Additionally, a plan to replace the Alfresco platform is in development. Assessment of VDW user needs will be conducted to assist in identifying an alternative with enhanced functionality, ease of use and facilitation of collaboration.
The SON concluded with a thank you to the Annual Conference Planning Committee including co-chairs Karen Coleman and Claudia Steiner, and special acknowledgment of Anand, Shah, MD, MS, and Kaiser Permanente Community Benefit Trust for their generous support of the conference. Dr. Horberg announced that the 2024 conference will be held on April 9 – 11 in Milwaukee, WI.
On Wednesday, February 22, a special interest session was held on navigating the new NIH data management and sharing policy. The intent of this new policy was to promote the sharing of scientific data to accelerate biomedical research discovery. A panel of experts including, Lawrence H. Kushi, ScD, Kaiser Permanente Division of Research, Andrea Burnett-Hartman, PhD, MPH, Senior Investigator at the KP Colorado Institute for Health Research and Alex Cramer, KFRI shared their knowledge and expertise to address how the new policy will impact research and proposal development. Fielding questions such as What research is subject to the new DMS policy? What scientific data needs to be shared? When does data need to be shared and for what period of time? and How do we select a repository?, the panel offered guidance to enable more efficient implementation of the policy within HCSRN member institutions.
Closing out the meeting was a plenary session entitled, How do Health Equity Investigators Navigate the Intersection of Scientific Inquiry and Personal Experience? that highlighted the experiences of scientists and research staff who both identify as members of communities that are typically studied in health equity research and conduct their own research within these communities. The panel included Kanetha B. Wilson, PhD; Center for Research and Evaluation Kaiser Permanente Georgia; Katherine Sanchez, PhD, LCSW; Baylor, Scott & White Research Institute; Leslie A. Wright, MA; Institute for Health Research Kaiser Permanente Colorado; Divya Subramaniam, PhD, MPH; Department of Health and Clinical Outcomes Research Saint Louis University School of Medicine; and was moderated by Karen J. Coleman, PhD; Department of Research and Evaluation, Kaiser Permanente Southern California. The panel discussed how they navigate the intersection of scientific inquiry and personal experience as well as experiences they have had with systematic implicit/explicit bias and how they have learned to overcome the impact of this bias on their work and careers.
|
|
|
2023 Annual Conference By The Numbers:
- 321 Registrants
- 106 Accepted abstracts/panel presentations
- 33 Posters
- 9 Ancillary Meetings
- 33 Sessions
- 15Annual Conference Planning Committee Members
- 36 Volunteers
|
|
|
|
2023 Conference Award Winners
|
|
HCSRN celebrated the work of their colleagues with six awards which were presented during the conference. The following recognitions were presented:
|
|
|
|
|
Hung Fu Tseng, Bradley K Ackerson,
Yi Luo, et al
Effectiveness of mRNA-1273 against
SARS-CoV-2 Omicron and Delta Variants Nature Medicine, 2022.
|
|
Multisite Collaborator of the Year
|
|
Ingrid Binswanger, MD, MPH, MS
Senior Clinician Investigator
Kaiser Permanente Colorado
The Association Between Buprenorphine Treatment Duration and Mortality: A Multi-
Site Cohort Study of People Who Discontinued Treatment
Collaborative Sites included in the study:
- Kaiser Permanente Colorado
- Kaiser Permanente Southern California
- Kaiser Permanente Northern California
- Kaiser Permanente Mid-Atlantic
- Henry Ford Health Systems
- Geisinger
- UMass/Meyers
|
|
|
|
|
|
Assiamira Ferrara, MD, PhD.
Research Scientist III
Director of the UPSTREAM Center
Nominated by:
KP Northern California
Julie Schmittdiel, PhD MA
Monique Hedderson
Rana Chehab
Tracy Lieu
University of Tennessee
Samantha Ehrlich
UC Davis
Susan D. Brown, PhD
|
|
Early Career Investigator
|
|
Apoorva Pradhan, MD, MPH
Staff Scientist
Geisinger
Abstract
Impact of a Multi-Faceted Best Practice Alert among Patients with Headaches seen in Primary Care
|
|
|
|
Poster Session 1 Winner
Predicting 30-Day Hospital Readmission Using Machine Learning
in a Non-hospital-based Integrated Healthcare System
Robert Neuman, MD
Cardiologist, Physician Program Director for Research
Kaiser Permanente Georgia
Rochele Heflin-Wiley, RN, BSN, MSA, CCM
Manager of Transition Care Management
Operation Support Services
The Southeast Permanente Medical Group, Inc.
Poster Session 2 Winner
A Review of the Literature and Publicly Available Data
on Medical Aid in Dying in the US
Geoffrey Kahn, PhD
Research Fellow
Henry Ford Health System
|
|
|
|
|
Alison Galbraith, MD, MPH
|
|
|
The Governing Board of the Health Care Systems Research Network (HCSRN) has undergone some change in the last few months. HCSRN bid farewell to Lois Lamerato, PhD and Alison Galbraith, MD, MPH. Dr. Lamerato represented the Henry Ford Health System (HFHS) and joined the Governing Board in 2012. She served as the liaison to the Virtual Data Warehouse from 2012 -2022. During her tenure, she worked on the very first VDW content areas-Tumor and Enrollment and served as the scientific lead for the VDW Tumor work group for nearly 12 years. Lois is enjoying semi-retirement since she left HFHS at the end of year. Dr. Galbraith accepted a new position as the Chief of the Division of Health Services Research in the Pediatrics Department at Boston Medical Center, and left Harvard Pilgrim January 31, 2023. Alison had served on the HCSRN Board since 2019 and led HPHC’s participation as a site in the HCSRN’s ACTION IV application.
The Board expresses deep gratitude and appreciation to Lois and Alison for their dedication and leadership.
|
|
|
Taking over as the HFHS liaison is Christine Neslund-Dudas. Chris is an associate research scientist in the Department of Public Health Sciences at HFH and Co-Leader of the Cancer Epidemiology, Prevention and Control Program within the Henry Ford Cancer Institute. She is a medical sociologist and holds an appointment in the Department of Epidemiology and Biostatistics at Michigan State University. Her research focuses on disparities across the cancer continuum for Black Americans, including cancer prevention, screening, etiology, and care delivery. Chris is the HFH Site-PI for the NCI funded PROSPR-Lung Screening Consortium and the PCORI funded Healthy Lungs pragmatic smoking cessation trial and Co-I on the NCI Connect for Cancer Prevention cohort study. Chris received her doctoral degree from Wayne State University in Detroit.
|
|
|
Christine Neslund-Dudas, PhD
|
|
|
|
|
Darren Toh, ScD has joined HCSRN as the Board Representative for Harvard Pilgrim. Darren is the DPM Endowed Professor in the Department of Population Medicine at Harvard Medical School and Harvard Pilgrim Health Care Institute. He is a pharmacoepidemiologist with an interest in the comparative safety and effectiveness research of medical products. His research focuses on 1) assessing the risks and benefits of medical products using electronic data collected as part of routine healthcare delivery, and 2) developing and applying privacy-protecting analytic methods to conduct multi-center studies in distributed data networks. Darren is Principal Investigator of the Operations Center of the FDA-funded Sentinel System, a congressionally mandated national medical product safety surveillance system. He also leads the Analytic Center of the Innovation in Medical Evidence and Development Surveillance (IMEDS) program. Darren received his doctoral degree in Epidemiology from the Harvard School of Public Health.
|
|
|
Transgender Health Research at Integrated Health Systems: Methods, Lessons, Needs
|
|
|
Michael Goodman, MD, MPH
Professor of Epidemiology
Director, MD/MPH program
Emory University School of Public Health
This article is based on Dr. Goodman’s presentation at the VIG Fall Midyear Meeting held on October 18, 2022 in Atlanta.
Transgender people are a large and diverse group that includes individuals whose assigned sex does not match their gender identity. Many transgender people may not self-identify based on binary definitions of man or woman; however, a person whose gender identity differs from male sex assigned at birth is often referred to as transfeminine and a person whose gender identity differs from female sex assigned at birth is referred to as transmasculine.
Transgender individuals sometimes seek medical gender affirmation, which may involve administration of hormone therapy, surgical changes of the chest and genitalia, or other procedures aimed at altering secondary sex characteristics. Although several medical organizations have issued recommendations for appropriate care of transgender patients, many unresolved issues in transgender health remain. Critical research priorities in transgender health include, for example, identification of the best ways to alleviate gender dysphoria (the feeling of distress when assigned sex does not match gender identity) and investigation of the most appropriate methods of monitoring transgender persons who receive hormone therapy. Addressing these research priorities requires high-quality longitudinal data on large numbers of transmasculine and transfeminine people followed for sufficient period of time. These considerations motivated the design of the “Study of Transition, Outcomes & Gender (STRONG)” a large cohort nested within integrated health systems.
STRONG initially was established in 2014 as a cohort of 6,456 transgender people ascertained from electronic health records of three Kaiser Permanente sites in Georgia and in Northern and Southern California. Eligible cohort members were identified using a combination of diagnostic codes and presence of confirmatory clinical notes. Each eligible STRONG participant was also categorized as transfeminine or transmasculine based on relevant diagnoses, procedures as well as evidence of in the text. Up to 10 cisgender male and 10 cisgender female Kaiser Permanente plan members were matched to each transgender participant on year of birth, race/ethnicity, study site, and enrollment year. To date the STRONG study provided data for over 30 publications covering a wide range of important transgender health issues.
More recently we expanded the cohort, which now includes nearly 40,000 transgender persons matched with 800,000 cisgender referents identified across four different integrated health systems. The expanded STRONG cohort provides data for a series of new analyses aimed at investigating occurrence of acute cardiovascular events in relation to gender affirming hormone therapy, examine mental health status of transgender people with specific focus on suicide attempts and cognitive decline, and characterize changes in clinical health indicators such as serum lipids, liver enzyme levels body mass index and bone density in relation to HT dose and duration.
Although the expanded cohort offers a plethora of new research opportunities, the greatly increased volume of the available data also presents a number of new challenges. Some research questions require new methods of systematically identifying patients who underwent diagnostic workup or treatment outside of the integrated health systems. For example, some genetic reports are only available as scanned files and converting information in these reports into electronic data may be time and resource-consuming. One of critical elements of gender affirming care is monitoring of hormone levels. Adding hormone levels to the list of variables available in the Virtual Data Warehouse will greatly facilitate research efforts. As the number of participants increased it has become impossible to rely on human review of clinical notes to ascertain and characterize study participants. Use of Natural Language Processing programs holds great promise; however, it is important to ensure that these programs can be uniformly deployed and tested across sites. These challenges can be viewed as barriers as well as opportunities. Overcoming these challenges will greatly advance research methodology and, if so, place integrated health systems in a unique position of providing much needed information about transgender health as well as health of other hard-to-reach understudied populations.
|
|
|
Summary of HCSRN Scientific Data Resources Forum Presentation October 11, 2022
|
|
|
The Kaiser Permanente Research Bank Cancer Cohort: A collaborative resource to improve cancer care and survivorship
|
|
|
Heather Feigelson, PhD MPH
|
|
|
Shauna Goldberg Scott MPH
|
|
|
Over the past 30 years, the cancer death rate has fallen significantly, primarily due to effective screening and improved treatment. By 2040, there will be an estimated 26 million cancer survivors in the US. Kaiser Permanente (KP) is an ideal environment to study the spectrum of cancer care and long-term survival because KP provides comprehensive medical care, including cancer care, to a large and diverse membership.
The KP Research Bank (KPRB), a research resource within KP, has collected biospecimens and surveys from over 400,000 adult members across all 8 KP regions and linked that information to medical record data. Within the KPRB, we have developed a cancer cohort to specifically address issues related to cancer survival, and to understand how genetic, lifestyle, and environmental factors impact cancer treatment, treatment sequalae, and prognosis. Cancer cases are identified through several methods: (1) a rapid case ascertainment algorithm, (2) direct outreach to cancer survivors, (3) in person recruitment and outreach to oncology, and (4) identification of prevalent and incident cancers among those already enrolled in the KPRB.
As of December 31, 2020, the cancer cohort included 65,225 cases (56% female, 44% male) verified in tumor registries. The largest group was diagnosed between 60-69 years of age (31%) and are non-Hispanic White (83%); however, 10,076 (16%) were diagnosed at ages 18-49 years, 4,208 (7%) are Hispanic, 3,393 (5%) are Asian, and 2,389 (4%) are Black. The median survival time is 14 years. Blood or saliva samples are available on 98% of the cohort, and genotyping of the cohort will be completed in 2023.
An important strength of the cancer cohort is the diversity with respect to age, race/ethnicity, and geographic location. An important weakness is the low response rate (less than 10%). Because the response rate was low, the cohort likely is not representative of all people diagnosed with cancer in KP. Nonetheless, the KPRB cancer cohort is a rich data resource that will enable research to improve understanding of treatment efficacy and lifestyle factors that contribute to long-term cancer survivorship.
The KPRB welcomes collaboration from investigators both within, and beyond, KP. For more information, visit the KPRB website: https://researchbank.kaiserpermanente.org/
For more information about the KPRB Cancer Cohort, please see our full publication: Feigelson HS, Clarke CL, Van Den Eeden SK, Weinmann S, Burnett-Hartman AN, Rowell S, Scott SG, White LL, Ter-Minassian M, Honda SAA, Young DR, Kamineni A, Chinn T, Lituev A, Bauck A, McGlynn EA. The Kaiser Permanente Research Bank Cancer Cohort: a collaborative resource to improve cancer care and survivorship. BMC Cancer. 2022 Feb 25;22(1):209. doi: 10.1186/s12885-022-09252-6. PMID: 35216576; PMCID: PMC8876075.
|
|
|
Mark your calendars for the 2024 HCSRN Annual Conference!
|
|
|
|
|